Ethanol Spills – Understanding the Animal
Over my twenty years in the hazardous materials cleanup industry, I have learned to think about every chemical as having a personality. You can even make the analogy that every chemical is a different animal. You may encounter the animal under different circumstances, but you can expect a lot of the same behavior from that guy every time.
In terms of distillery safety, chemical and physical hazards can also be thought of as entities with a personality.
Carbon monoxide: a stealthy, silent, slithering animal. It has no smell, no color, and slowly suffocates you by preventing oxygen from binding to your hemoglobin.
A great white shark could be compared to a shock sensitive chemical. It’s rare, but you don’t want to encounter it. If you do, it could have explosive and fatal repercussions.
Safety jocks define a product’s personality with a set of characteristics called chemical and physical properties – things like pH, solubility, flammability. Many of these will be listed on the safety data sheet (SDS), but you have to know what to look for and know what these behavioral traits tell you. The properties of a chemical material are given in a shorthand version, a code if you will. These may be a keyword or a numerical value.
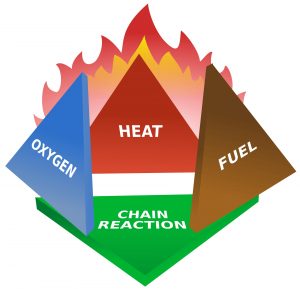
The classical view of the fire tetrahedron requires fuel, oxygen, a source of ignition, and a method of sustaining a fire. But only fuel, oxygen, and an ignition source are necessary for an explosion.
Since I’m writing specifically about ethanol, let’s consider behaviors related to one of ethanol’s hazardous properties – its ability for the vapors to catch fire. For the vapors of a substance to catch fire we need several conditions to co-exist. I’m going to go beyond the old “fire tetrahedron” of fuel, oxygen, ignition source, and ongoing reaction or sustainability.
Let’s consider this scenario: a high proof spirit that spilled in a production area. Then let’s describe a proper response, as if we were hazmat responders who don’t work with the product every day. In our scenario, the fact that this cleanup team has never before responded to an ethanol spill doesn’t really matter, because this team will know what to expect from ethanol by first reviewing the chemical and physical data in the SDS, and then by applying that information to their knowledge of hazard control techniques.
In a chemical spill, the first thing the responders do is to secure the scene so there aren’t incidental exposures. In hazmat responder parlance, we’d say “Keep the bad boy in and the stupid people out.” Translated: “Contain the hazardous material and keep non-essential people away for their own safety.” It doesn’t literally mean untrained people are stupid, it is just jargon for “control the situation.”
After that, we “size up” the problem by: a) determining what product is spilled and how much, b) understanding the behaviors of said product, and c) developing a response plan to mitigate hazards so cleanup can commence. A fundamental rule of hazmat response is “don’t make it worse while trying to make it better.” And another useful concept, if one can do it safely, is to control the source of a spill first, the control where the spill is going. Again, chemical and physical behavior given in the SDS will cues us on the best course of action.
So, we know it is ethanol. Let’s say that a third of a 250-gallon IBC tote of 190-proof GNS spilled due to operator error of a valve – 80 gallons of liquid is a big spill on a flat floor. It is 68ºF in the distillery at the time. We evacuate the area except for essential personnel. We make a decision as to whether or not to call the municipal response team or deal with it ourselves, depending on training and resources. It would be prudent in this case to call for fire department backup right at the beginning.
Moving on to item “b,” we consider the hazardous properties of ethanol at this concentration. First, the vapors are ignitable, if the right conditions are met. More on that in a moment. Next, the liquid itself is flammable. Thirdly, are there other hazards, say respiratory hazards, operating machines or processes, wastewater pollution if the alcohol goes down the drain, or difficult access and egress that endangers the cleanup crew. We do anything we can do immediately to stop the worsening of the problem if we can do it without jeopardizing ourselves. For example, perhaps we close the valve that was left open or turn off the boiler in the next room.
Starting with the ignitable vapors issue, we consult the SDS and find numerical values for vapor pressure, vapor density, lower and upper explosive limits, and flashpoint. Allow me to explain what each of these is, and why each is significant.
Vapor Pressure
Vapor pressure (v.p.) can be the hardest of these to explain. Technically, it is a measure of the equilibrium between liquid and gas states. If we allow a volatile solvent to evaporate from a beaker placed into an airtight box, vapors will naturally accumulate until that equilibrium is reached, and from that point onward there will be a percentage of solvent in the vapor state, and a percentage as a liquid. If the temperature, volume and pressure of the box stay fixed, the ratio of vapor to liquid will not change. A simplified way to remember v.p. is to ask yourself “How badly does it want to go from the liquid state to the gas (vapor) state?”
The v.p. is typically given in units of mmHg, a barometric measure where 760 mmHg equals one atmosphere of pressure. If the v.p.=0, then that substance does not evaporate at all. Think of say, a rock. If the v.p.>760 mmHg, then that stuff will be entirely a gas at normal conditions. An example would be butane with a v.p.=1,650. You can have it as a liquid under pressure in a lighter, but as soon as it comes out of its container into room temperature and ambient pressure, it instantly and completely vaporizes.
What happens if the v.p. is between zero and 760 mmHg? In that case, the liquid partially evaporates until it reaches the equilibrium between liquid and gas states. At the temperature of our facility, 68ºF (20ºC), water has a vapor pressure of 17.5 mmHg and the ethanol is at 44.6. This makes sense, because we know water evaporates slowly over time and that ethanol evaporates faster.
Okay, so you’re thinking, only so much ethanol can evaporate, because some will stay liquid to maintain equilibrium. Not so fast, assuming our spill was in a somewhat open area of the distillery, the ethanol will keep evaporating and keep evaporating, because it never achieves its equilibrium while evaporating – the spill isn’t occurring in a laboratory box of fixed volume. Given enough time, all of this spill will evaporate, even the water.
So, we’ve got two pieces of information: ethanol vapors are forming above the liquid spill and that is an awfully big puddle.
Vapor Density
A logical follow-up question is, “Ok, where are these alcohol vapors?” That question we answer with vapor density (v.d.). Vapor density is to gases what specific gravity is to liquids. The v.d. relates the density of a gas or vapor against that of air, air being normalized at 1.00. Ethanol’s v.d. is 1.59, so it’s vapors are heavier than air. (If your SDS doesn’t list a v.d. for a substance, take the molecular weight of the chemical and divide by the average molecular weight of air, 29.) Now we know those vapors are about as heavy as carbon dioxide – v.d.=1.52. In still air ethanol vapors will “creep” along the floor, go down stairwells, and accumulate in lower portions of a sewer system.
Lower Explosive Limit
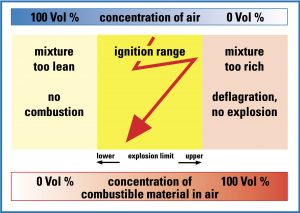
The “sweet spot” for an ethanol explosion occurs between 3.3 – 19% vapors in air. (Source: ControlGlobal.com)
We now know to expect there to be ethanol vapors and we know they will mostly be at lower elevations unless the air is turbulent. Now let’s consider if these vapors are explosive. That’s where we look at the lower explosive limit (LEL) and the upper explosive limit (UEL). We’re usually more concerned with the LEL, because by the time we get to the UEL we may be impaired by the chemical or already blown up. In the gruesome humor of hazmat responders, this is called, “First ya die, then yu’r cremated.”
All jokes aside, the LEL for ethanol is when you achieve a mixture of 3.3% alcohol vapors in air. At this point, there’s enough vaporized fuel (ethanol) and still enough oxygen in the air to support combustion. Above this concentration we’re in a dangerous flammable state all the way to 19% alcohol vapors in air, the UEL. Most chemicals have a UEL less than 100% because at some point the vapors have displaced enough air that there is insufficient oxygen to support an explosion. At ethanol’s 19% UEL, you can expect less than about 17% oxygen to remain, so by this point you may already be unconscious.
Flash Point
The only thing keeping us from having an explosion is whether the vapors are warm enough and if the vapors have found a source of ignition. You may know that in very cold climates, there is a temperature at which your gasoline vehicle won’t start because not enough vapors are forming at very frigid temperatures. That minimum temperature at which flammable vapors explode is called the flashpoint (f.p.).
Our SDS says the f.p. For ethanol is 55.4ºF. So, if our vapor is above that temp, and greater than a 3.3% concentration in air, and has found its way to a source of ignition – game over.
Miscibility and Specific Gravity
One other thing we would like to know about ethanol is whether it mixes completely with water (is miscible) or does not mix with water (immiscible). Hand in hand with miscibility is the specific gravity (s.g.) of the liquid. Where v.d. Compares a substance with air, s.g. uses water as the standard at 1.00 grams per milliliter (g/mL). A gravity less than 1.00 and the material may float on water; a gravity greater than 1.00 and the material may sink.
If you have a material that floats and is immiscible, like lacquer thinner (principle constituent is toluene), applying water during a spill creates a bigger puddle with great surface area. The lacquer thinner is on top of that puddle and now has more chance than ever to evaporate. Although lacquer thinner is a variable mixture, depending on manufacturer, typical specs include: v.p. > 100 mm Hg, v.d. = 2.7, s.g. = ± 0.8 g/mL, LEL = 1.8%, and f.p. = 3-20ºF, so trying to wash away a lacquer thinner spill with water only increases the chances of a hazardous explosion.
If you’re in this spirits industry, you already know ethanol is miscible with water. It does not form a bilayer system, the way oil and vinegar do, or lacquer thinner and water. In fact, there is no mixture of ethanol and water that doesn’t fully mix, so we can say ethanol is infinitely miscible with water.
Knowing the miscibility gives us some good news and some bad news. The good news is that the spill treatment might involve washing the spill into a safer area, like an open field, if we are lucky enough to have that circumstance. The bad news is we might wash the spill into a sewer where vapors continue to concentrate and can explode if they encounter a sewage lift pump or similar ignition source, or we may wash it into surface water killing fish and reptiles.
Now, if we look comprehensively at all of these behavioral descriptors for ethanol, we know it mixes with water, its vapors are flammable at room temperature, the vapors tend to stay close to the floor and will creep into low lying areas. We know the longer the spill goes untreated, the bigger the spill will become, the more ethanol will evaporate, and the chance of explosion will grow. So in this case, we would work aggressively to: a) eliminate sources of ignition a.s.a.p. – thermostats, pumps, boiler igniters, and Heaven forbid, a direct fired kettle system, and b) reduce vapors by stopping the source of the leak, absorbing spilled liquid, flushing into a safer place, or rapidly increasing ventilation without introducing the chance for electrical ignition,
This sort of thought exercise is called “hazard assessment” or “emergency planning.” In activities involving high proof materials at your distillery, you need to have previously thought through these sorts of possibilities and already worked out your plan of attack. Only then can you respond effectively to minimize the chance of a terrible outcome.
Whalen Insurance has teamed up with Markel Specialty Commercial to offer craft distillers superior coverage tailored to the distillery insurance needs of the industry. Click HERE to find out more.




